USP 800 Oncology PPE Requirements & Guidelines:The Ultimate Protection for Hazardous Drug Handling
Mastering USP 800 PPE Requirements. Medtecs provides a synergistic defense with ASTM D6978 Certified N1000 Chemo Gloves and CoverU™ Impervious Gowns.
Verified protection for healthcare personnel handling antineoplastic drugs including Cyclophosphamide, Thiotepa, and Cisplatin. Meets Occupational Safety and Health (OSHA) and Centers for Disease Control and Prevention (CDC) guidelines.
Visualize the Invisible Threat. Standard personal protective equipment fails against Carmustine in <27 mins. Medtecs CoverU™ polyethylene-coated polypropylene gowns provide a >480 min barrier against 19 NIOSH hazardous drugs.
I am a…
The Hidden Danger: Pathology Risks & Invisible Permeation
In Healthcare Settings, 'waterproof' does not mean 'chemical-proof'. Permeation is a molecular process where hazardous drugs diffuse through standard personal protective equipment—invisible to the naked eye, but devastating to long-term health per Occupational Safety and Health (OSHA) guidelines.
Carcinogenicity
Increased leukemia risk from cumulative exposure. Meta-analysis shows 1.7x increased risk in oncology nurses.
Lawson et al., Am J Ind Med, 2012
Teratogenicity
2.3x higher spontaneous abortion rate documented in healthcare workers handling antineoplastic drugs.
Dranitsaris et al., J Oncol Pharm Practice, 2005
Chromosomal Aberrations
Genetic damage observed in 75% of nurses handling cyclophosphamide without proper PPE.
NIOSH Publication 2004-165
Organ Toxicity
Liver/Kidney damage from dermal absorption. HD contamination detected on 100% of pharmacy surfaces.
Connor TH, AJHP 1999
Synergistic Risk Alert: Combination Regimens
In complex regimens (e.g., CHOP or FOLFIRINOX), the synergistic toxicity of combined agents on healthcare workers remains understudied. Our permeation data (tested per ASTM protocols) covers both individual agents and common combination protocols, providing verified protection when multiple antineoplastic drugs are handled simultaneously.
Aerosolization: The Invisible Migration Path
Micro-aerosols generated during vial spiking or needle withdrawal can remain suspended in the air for hours. Without proper gowning, these invisible particles settle on skin and scrubs, migrating home to families. Standard respiratory protection alone cannot prevent dermal contamination from settled aerosols.
Vial Spiking
Generates aerosols during reconstitution
Needle Withdrawal
Creates micro-droplets at needle tip
Priming IV Lines
Releases drug into ambient air
Emerging Risks: ADCs, CAR-T & Precision Medicine
Future-proofing for next-generation therapies
Antibody-Drug Conjugates (ADCs)
ADC ReadyADCs targeting HER2/TROP2 combine monoclonal antibodies with extremely potent cytotoxic payloads that require next-generation protection exceeding standard chemo ratings.
Viral Vector Protection (CAR-T)
Viral Vector ReadyFor CAR-T cell therapy preparation involving viral vectors, our gowns provide a dual barrier: preventing viral escape (ASTM F1671) while maintaining a sterile field.
Biologic Integrity & mRNA Protection
Low-linting materials protect mRNA Vaccines from human DNA contamination per USP <797>. Critical for emerging biologic therapies.
Pharmacogenomics & Targeted Dosing
As biomarker testing enables more potent dosing regimens, chemotherapy-tested gowns (per ASTM permeation standards) become the only safe baseline for personalized oncology.
Chemical Barrier
Permeation Tested
Tested against 19 chemotherapy drugs per ASTM standards
Viral Barrier
ASTM F1671
Blood-borne pathogen penetration resistance
ASTM D6978 permeation data for Carmustine at 35°C (body temperature simulation)
"Don't trust generic labels. Standard gowns fail against Carmustine in <27 minutes. Medtecs CoverU™ ensures worker protection for >480 minutes, covering the entire shift from admixture to Decontaminating."
The Hierarchy of Controls: Integrating PPE with C-PECs
Effective safety is a system. Engineering controls are the first line of defense—from the Biological Safety Cabinet to the patient bedside. Each layer must work in harmony to protect healthcare personnel per USP General Chapter <800> requirements.
Static Dissipation (ESD) Properties
Our proprietary fabric incorporates static-dissipative properties, reducing the risk of particulate attraction and Electrostatic Discharge (ESD) events that could compromise sensitive electronic lab equipment in cleanroom supplies environments.
Laminar Airflow Optimization
Bulky, loose-fitting cotton gowns create turbulence in the laminar airflow of a Class II BSC. Medtecs' streamlined fit minimizes air disturbance, maintaining the ISO 5 environment critical for sterile compounding in C-PEC environments.
Healthcare Worker
Protected by ASTM D6978 certified personal protective equipment
Primary (C-PEC)
Secondary (C-SEC)
Final Barrier (PPE)
ISO Classification & HEPA Requirements
| Location | ISO Classification | Air Changes/Hour | Pressure Differential |
|---|---|---|---|
| C-PEC Interior | ISO 5 | N/A (unidirectional) | Negative to room |
| C-SEC / Buffer Room | ISO 7 | ≥30 ACPH | Negative to adjacent |
| Anteroom | ISO 7 or 8 | ≥20 ACPH | Positive to C-SEC |
Per USP Chapter <800> and USP Chapters <797> and <800> requirements. Low-linting cleanroom supplies are essential to protect HEPA filters and maintain ISO classified rooms integrity.
Chemical Compatibility: Decontamination Agents
Tested against rigorous disinfectants per the ‘Deactivate, Decontaminate, Clean, Disinfect’ cycle:
| Cleaning Agent | Formula | Status | Use Case |
|---|---|---|---|
| Sodium Hypochlorite (Bleach) | NaClO | ✓ Compatible | Primary decontaminant |
| Quaternary Ammonium | QAC | ✓ Compatible | Surface disinfection |
| Hydrogen Peroxide | H₂O₂ | ✓ Compatible | Vapor decontamination |
| Isopropyl Alcohol | IPA | ✓ Compatible | Equipment wipe-down |
Medtecs gowns maintain integrity during rigorous decontaminating procedures, ensuring protection throughout the entire cleaning cycle in Hospital Environments.
Synergizing PPE with Engineering Controls:
USP <800> Compliant Hand Protection Solutions
Chemotherapy-tested gloves are the most frequently contacted PPE in hazardous drug handling. USP <800> mandates Double Gloving for all compounding and administration tasks. Medtecs N1000 Nitrile Series provides ASTM D6978 tested protection as the foundation of your hand protection protocol.
⚠️ Procurement Warning: Not All "Exam Gloves" Are Equal
Many facilities mistakenly use standard ASTM D6319 exam gloves for chemotherapy handling. This is non-compliant with USP <800>. Only gloves tested to ASTM D6978(chemotherapy-specific permeation standard at 35°C body temperature) are acceptable for antineoplastic drugs.
N1000 Chemo Gloves: Precision and Protection
Technical Specifications

Finger-Textured Design
Why N1000? Standard gloves fail as skin heats up. Medtecs N1000 series is tested at 35°C (Body Temperature Simulation) rather than standard room temp (23°C), ensuring the ASTM D6978 chemical barrier holds firm during real-world oncology operations.
Precision grip on glass vials and syringes without compromising the chemical barrier. Critical for Containment Aseptic Compounding Isolators where tactile feedback is essential.
Micro-Textured
Fingertip Pattern
4-5 mil
Optimal Thickness
ASTM D6978 Breakthrough Times
Tested at 35°C (body temperature simulation)
| Drug | Breakthrough | Status |
|---|---|---|
| Carmustine Highest permeation challenge | > 30 min | Pass |
| Thiotepa High lipophilicity | > 30 min | Pass |
| Cyclophosphamide Common alkylating agent | > 240 min | Pass |
| Cisplatin Nephrotoxic platinum compound | > 240 min | Pass |
| Doxorubicin Anthracycline cardiotoxin | > 240 min | Pass |
| Paclitaxel Taxane microtubule inhibitor | > 240 min | Pass |
| 5-Fluorouracil Antimetabolite | > 240 min | Pass |
| Methotrexate Folate antagonist | > 240 min | Pass |
| Etoposide Topoisomerase inhibitor | > 240 min | Pass |
Note: Carmustine and Thiotepa are the most challenging due to their small molecular size and high lipophilicity. > 30 min is the minimum ASTM D6978 requirement; Medtecs N1000 exceeds this threshold.
The 35°C Advantage: Unlike standard gloves tested at room temperature (23°C), Medtecs N1000 series is tested at 35°C (Body Temperature Simulation). This ensures the chemical barrier remains intact under actual usage conditions, providing a higher safety margin for oncology pharmacists.
USP <800> Double Gloving Protocol by Scenario
Recommended chemotherapy gloves configuration for healthcare personnel
| Scenario | Inner Glove | Outer Glove | Change Frequency |
|---|---|---|---|
| Sterile Compounding (USP <797>/<800>) | N1000 (Chemotherapy-tested) under gown cuff | ASTM D6978 compliant glove over cuff (sterile-ready options available) | Every 30 min or immediately if compromised |
| Non-Sterile Compounding | N1000 (Size M) under gown cuff | N1000 (Size L) over gown cuff | Every 30 min or between patients |
| Administration (Infusion Nurses) | N1000 tucked under gown | N1000 extending over gown cuff | Between each patient |
| Spill Response (>5ml) | N1000 Nitrile (chemical barrier) | Utility-grade Chemical Resistant Glove | After spill cleanup complete |
Pro Tip: Using a larger size for the outer glove (e.g., inner M + outer L) reduces hand fatigue during extended compounding sessions—a practice endorsed by experienced oncology pharmacists.
Choosing the Right Barrier Protection:
Medtecs Solution Matrix: Tiered Protection for Every Role
Pharmacy Directors need a tiered approach. From pharmacy techs compounding daily to spill response teams handling waste containment systems—choose the right protection level based on drug categories and workflow requirements.

Tier 1
Compounding
Tier 2
Surgery/HIPEC
Tier 3
Spill/Waste
IL-4036YKTP (Yellow)
Oncology Pharmacists, Pharmacy Techs
Specifications
Engineered PPSB+PE Laminate: Moisture-Vapor Breathable Technology (MVTR) optimizes thermal comfort while maintaining full barrier protection against oral chemo and routine admixtures.
Use Case
Daily Admixture in C-SCA, Handling Oral Chemotherapy, Virtual Hybrid IV Certification.
Key Features
Permeation Test Results*
🧤 The Perfect Pair: Gown + N1000 Gloves
Under-and-Over Technique: Larger outer size reduces hand fatigue
IL-4063WKTP (White)
Surgeons, OR Nurses
Specifications
63gsm Heavy Duty, AAMI Level 4, ASTM F1671 Viral Barrier.
Use Case
HIPEC Surgery, Trauma with Chemo Patients, High-fluid procedures.
Key Features
Permeation Test Results*
🧤 The Perfect Pair: Gown + N1000 Gloves
Under-and-Over Technique: Maintains sterile field + chemo protection
IL-3063WEHTP (Coverall)
EVS Staff, Spill Response Teams
Specifications
Type 5-B/6-B, Hooded, Taped Seams, Full body coverage. Compatible with Daniels' Sharpsmart protocols.
Use Case
Cytotoxic Drug Spills (>5ml), Waste Disposal, Disposing of HD. 100% Polyolefin material ensures Eco-Friendly Incineration (No toxic fumes).
Key Features
Permeation Test Results*
🧤 The Perfect Pair: Gown + N1000 Gloves
Under-and-Over Technique: Outer protects against sharps (broken glass)
Mandatory Add-ons (USP <800>)
Product Specification Comparison Table
| Specification | IL-4036YKTP | IL-4063WKTP | IL-3063WEHTP |
|---|---|---|---|
| Product Type | Isolation Gown | Isolation Gown | Coverall |
| Weight (gsm) | 36 | 63 | 63 |
| Material | PPSB+PE | PPSB+PE | SMS+PE |
| AAMI Level | Level 3 | Level 4 | N/A |
| Permeation Resistance (19 drugs)* | >480 min | >480 min | >480 min |
| ASTM F1671 (Viral) | — | ✓ Pass | ✓ Pass |
| Type 5-B/6-B | — | — | ✓ Yes |
| Heat-Sealed Seams | ✓ Yes | ✓ Yes | ✓ Yes |
| Primary Use Case | Compounding, Administration | HIPEC, Surgery, Trauma | Spill Control, Waste Disposal |

Pharmacy Zone: Compounding Personnel
Maximum Exposure Risk
Compounding personnel face the highest exposure risk in the chemotherapy supply chain. Work shifts often exceed 4 continuous hours with direct contact to undiluted cytotoxic drug concentrates. Standard gown breakthrough time is only 27 minutes — far below a single shift duration.
Exposure Time vs Breakthrough Time
Conclusion: Standard gowns fail within 27 minutes, leaving 3.5-7.5 hours of unprotected exposure.
HEAD-TO-TOE GEAR
Isolation Gown: IL-4036YKTP-CM
AAMI Level 4, Taped Seams, Yellow
Carmustine breakthrough > 480 minutes
Double Chemotherapy-Tested Gloves
Inner: N1000 under cuff | Outer: ASTM D6978 compliant over cuff
Shingle Effect layered protection | Sterile-ready configurations available
NIOSH-Compliant Respiratory Protection
Per facility protocol for aerosol protection during compounding
Compatible with all major respirator brands
Double Shoe Covers
Inner + Outer for contamination control
Recommended Product Model
Knowledge Base for Compounding Pharmacists:

Nursing Zone: Oncology Administration
Chemical Hazard
Lower drug concentration than compounding, but protection still required for IV connection/disconnection leaks
Biological Hazard
Patient vomit, excreta, and blood contain active drug metabolites for 48 hours post-treatment
Why Nurses Need AAMI Level 4
ASTM F1671
Viral barrier certification — protects against blood-borne pathogens (HIV, HBV, HCV)
Chemotherapy Drug Permeation
Tested against 19 chemotherapy drugs per ASTM permeation standards
Full-Back Design
Prevents back contamination when moving or turning in patient care areas
Daily Exposure Scenarios for Nurses
IV Line Operations
Micro-droplet splashes when connecting/disconnecting IV lines, especially accidental leaks from Closed-System Transfer Devices (CSTD)
Patient Care
Handling post-chemo patient vomit, changing diapers, cleaning excreta — these body fluids remain cytotoxic for 48 hours
Accidental Leak Response
Extravasation during administration requires immediate response and PPE change
Comorbidity Management
Oncology patients often present with COPD, require Insulin Use, or need Continuous Glucose Monitoring alongside chemo protocols
HEAD-TO-TOE GEAR
Isolation Gown: IL-4063WKTP or IL-4036YKTP-CM
AAMI Level 4, Full-Back Coverage
Tested against 19 chemotherapy drugs | ASTM F1671 viral barrier
Double Gloves
N1000 tucked under + over gown cuff
Change every 30 minutes or when visibly contaminated
Eye Protection Goggles
Splash protection during IV manipulation
Home Infusion: Portable Compliance
Emerging MarketFor Home Infusion Nurses visiting patients, our lightweight packaging and easy donning design ensure USP <800> compliance even in uncontrolled home environments. The portable kit fits in a standard medical bag, enabling proper protection outside traditional Hospital Environments.
Compact Packaging
Single-use sealed pouches
Easy Donning
5-minute self-gowning procedure
Waste Protocol
Includes disposal bag
>480
min breakthrough
Dual
barrier certified
Cross-Contamination Alert: Insulin Injection Risk
When transitioning from chemo administration to insulin injection, a contaminated gown cuff can transfer HD contamination residue to the diabetic patient's injection site. Our Elastic Thumb Loops and strict cuff protocols prevent this cross-contact, protecting patients with Continuous Glucose Monitoring needs.
Recommended Product Models
Following USP 800 guidelines for PPE is essential for maintaining a culture of safety. Our tiered protection matrix ensures that every role—from compounding in the cleanroom to bedside administration—meets the most stringent USP 800 ppe requirements.
Essential Guides for Oncology Nursing:

EVS Zone: Spill & Waste Response
Unknown Dose Risk
Environmental services personnel often face the highest "unknown dose" risk. They may not know the specific composition, concentration, or exposure duration of spills. Cleanup may require kneeling or contact with large volumes of liquid, placing higher demands on PPE hydrostatic pressure resistance.
Why EVS Needs Taped Coveralls
Kneeling Work
Floor spill cleanup requires kneeling — knees under pressure, standard seams will permeate
Taped Seams
Taped seams provide higher hydrostatic pressure resistance, preventing wet-through penetration
Coverall Design
Coveralls have no waist seam, ensuring full-body gap-free protection
High-Risk EVS Work Scenarios
Highest Risk
Large Spill Cleanup (>30mL)
Drug spills exceeding 30mL require Spill Kit use, may require kneeling to absorb large liquid volumes
High Risk
Contaminated Restroom Cleaning
Restrooms used by chemo patients — toilets and floors may have drug metabolite contamination
Medium-High Risk
Medical Waste Handling
Collecting and transporting chemo waste containers — packaging damage may expose contents
HEAD-TO-TOE GEAR
Coverall: IL-3063WEHTP
Coverall, Level 4, Taped Seams
Full-body design with no waist gaps
Face Shield
Full face splash protection
Required for spill cleanup
Heavy-Duty Chemical Gloves
Utility-grade chemical resistant over N1000
Double layer: Inner N1000 + Outer heavy-duty gloves
Impervious Boot Covers
Impervious boot covers
Must extend to mid-calf
Waste Management & Disposal Protocol
Trace Contaminated (Yellow)
- Worn PPE (gowns, gloves, shoe covers)
- Empty vials (<5ml residue)
- Sharps (needles, syringes)
- IV tubing and bags
Bulk Hazardous (Black)
- Spill cleanup materials
- Vials with >5ml residue
- Large volume drug waste
- Heavily contaminated PPE
Spill Response: Equipment Selection Guide
For Cytotoxic Drug Spills, select appropriate PPE based on spill volume per USP <800> safety precautions:
Primary PPE:
IL-4036YKTP (Yellow Gown)
Gloves:
Double N1000 gloves
Additional:
Eye protection goggles, NIOSH-approved respirator
Action:
Absorb with approved kit, double-bag waste
Primary PPE:
IL-3063WEHTP (Coverall)
Gloves:
Double N1000 + chemical outer gloves
Additional:
Face shield, NIOSH-approved respirator or PAPR, shoe covers (2 pairs)
Action:
Evacuate area, use full spill kit, contact EVS
Eco-Friendly Disposal: Clean Incineration
ESG CompliantConstructed from 100% polyolefins (PE/PP), our gowns incinerate cleanly, releasing only water vapor (H₂O) and carbon dioxide (CO₂)—no dioxins or furans. This reduces the environmental footprint of your hazardous waste stream while meeting EPA incineration requirements.
Recommended Product Model
Safety Protocols for EVS & Spill Management:
- Master USP 800 PPE Strategies for Safe Spill Control
- Mastering Environmental Safety with USP 800 Decontamination
- Mastering Safe Disposal of Hazardous Waste in Healthcare
Comprehensive Shield: Respiratory Protection & Critical Protocols
A gown is not enough. Hazard Communication standards require full mucosal protection— eyes, nose, and mouth must be shielded from aerosols and splashes. Per Occupational Safety and Health (OSHA) and USP Chapter <800> requirements, respiratory protection is mandatory when handling hazardous drugs outside of a C-PEC.
NIOSH-Approved Surgical Respirator
- NIOSH-approved (N95/P100 class)
- Fluid resistant
- Adjustable nose clip
- Protects against airborne particles
Elastomeric Half-Mask + Multi-Gas Cartridge
- Reusable design
- Multi-gas cartridge (OV/AG)
- Silicone face seal
- Protects against vapors AND gases
Face Shield + Eye Protection Goggles
- Full face coverage
- Anti-fog coating
- Over-glasses compatible
- Impact resistant
Safe Donning & Doffing Procedure
Proper Safe-Handling Adherence during PPE donning and doffing is critical. Doffing is the highest contamination risk phase per Hospital Policies and training courses requirements.
The Critical Glove-Cuff Interface
The USP <800> "Shingle Effect": Ensuring liquids flow OVER, not INTO, your protection
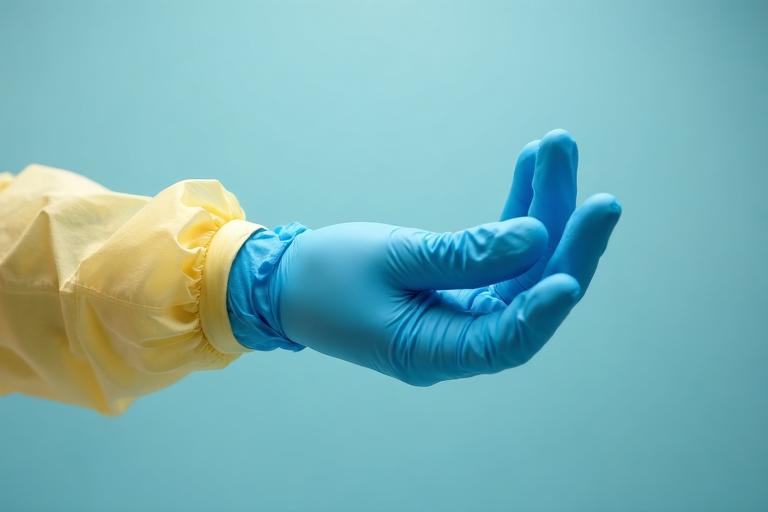
Proper "Under & Over" Technique: Inner blue glove tucked under yellow cuff, outer glove overlapping cuff
Inner Glove
Blue N1000 on skin, wrist exposed
Gown Cuff
Yellow knit cuff pulled OVER blue glove
Thumb Loop
Optional loop secures cuff position
Outer Glove
Blue N1000 pulled OVER yellow cuff completely
The "Under & Over" Principle
Inner glove UNDER gown cuff → Outer glove OVER gown cuff. This creates a “shingle” pattern where any liquid contamination flows down and away, never reaching skin through gaps.
Outer glove overlaps gown cuff by 2+ inches
Gap between glove and cuff exposes wrist
Donning (Putting On)
Hand Hygiene
Perform thorough hand washing before donning PPE
First Gloves (N1000)
CRITICALDon inner chemotherapy gloves - tuck under gown cuffs
Gown (IL-4036YKTP)
CRITICALDon isolation gown - ensure knit cuffs cover inner gloves
Secure Ties
Fasten neck and waist ties for full back closure
Second Gloves
CRITICALDon outer gloves OVER gown cuffs (overlap method)
Respirator/Face Shield
Don NIOSH-approved respirator or elastomeric mask + eye protection
Doffing (Removing) - HIGH RISK
Remove Outer Gloves
HIGH RISKPeel off and invert to contain contamination
Remove Gown
HIGH RISKUntie, roll contaminated side inward, dispose
Remove Face Protection
Carefully remove shield/goggles from behind
Remove Respirator
Remove by straps only - do not touch front
Remove Inner Gloves
HIGH RISKFinal barrier removal - invert and dispose
Hand Hygiene
Immediate hand washing post-doffing
Critical Safety Note
Particulate respirators (N95 class) do NOT protect against vapors or gases. When handling spills or working with volatile hazardous drugs, an Elastomeric half-mask with appropriate multi-gas cartridge is MANDATORY per NIOSH and USP <800> guidelines.
Standards for Respiratory Protection:
Building a Culture of Compliance: Training & Documentation
Compliance isn’t a checkbox—it’s a culture. Whether your facility manages documentation via manual logs or software like Simplifi 797, Medtecs supports your audit readiness for Occupational Safety and Health (OSHA) and State Board of Pharmacy requirements.
Thermal Mapping: Comfort → Reduced Compounding Errors
Heat stress in the cleanroom is a primary driver of protocol fatigue and compounding errors. Our breathable back panel design reduces core temperature retention by up to 40%, directly linking Comfort to Reduced Compounding Errors.
Standard PE Gown
High heat retention → Fatigue
Medtecs Breathable
Heat dissipation → Vigilance
Performance Dashboard
Track Compliance Rates across departments with real-time analytics per Hospital Policies
Observation Tool
Monitor Donning/Doffing procedures and identify training gaps for Safe-Handling Adherence
Audit Readiness
CoC & Test Reports ready for State Board of Pharmacy, JCI, and OSHA inspections with full compliance documentation
Training Alignment
Aligned with Medisca / PCCA Standards, Virtual Hybrid IV Certification, and training courses requirements
Aligned with Professional Standards
Compounding Compendium
Best practices for hazardous drug handling aligned with industry standards
American College of Apothecaries
Professional pharmacy standards and Expert Panel Discussion guidelines
Simplifi 797
Documentation management and compliance tracking software integration
Pharmacy Law Compliance
State Board of Pharmacy requirements and regulatory compliance documentation
Full Lot Traceability via QR Code
Every Medtecs gown features a QR code linking to its specific Lot Certificate of Compliance (CoC), ensuring full traceability from raw material to end-user for JCI Audit requirements. Scan → Verify → Document in seconds.
OSHA Compliant

Procurement Zone: Decision Makers
The Chain Impact of Procurement Decisions
As procurement decision makers, your choices directly impact the organization's compliance status, employee safety, and legal liability. A few dollars difference in gown price may seem minor, but a single occupational exposure lawsuit can cost hundreds of thousands of dollars.
Five Core Considerations for Procurement
Compliance Risk
Regulatory Exposure
Common Problem
USP <800> audit failures, fines, license revocation risk
Medtecs Solution
Complete ASTM D6978 test reports, Nelson Labs third-party verification documents
Supply Chain Stability
Geopolitical Resilience
Common Problem
Single-source disruption risk, geopolitical impact
Medtecs Solution
Medtecs 3-country production base (Philippines/Cambodia/China), TAA compliant
Total Cost of Ownership
TCO Analysis
Common Problem
Low-cost products tear requiring replacement, employee compensation costs
Medtecs Solution
>480 min breakthrough = single-gown full-shift use, reduced legal risk costs
Lot Traceability
Audit Documentation
Common Problem
JCI audit requirements, supplier documentation management difficulties
Medtecs Solution
QR Code traceability system, batch CoC online verification
Employee Health Protection
Workforce Safety
Common Problem
Occupational exposure claims, employee turnover rate
Medtecs Solution
Breathable design reduces heat stress, improves wear compliance rate
Total Cost of Ownership (TCO) Comparison
Hidden Costs of Low-Price Products
Medtecs CoverU™ TCO Advantages
Ready to Evaluate Your PPE Supplier?
Request complete product specifications, test reports, and pricing proposals
Strategic Resources for Procurement & Facilities:
- The Definitive Guide to USP 800 PPE Compliance for Oncology Facilities
- Essential Steps for USP 800 PPE Procurement
- Global Perspectives on USP 800 PPE in Oncology
- The Business Case for Safety: Justifying Investment in USP 800 PPE
- Essential Steps for Future-Proofing Your Oncology Facility with PPE Standards
Procurement & USP <800> Checklist
Prepare for your next JCI Audit or Centers for Disease Control and Prevention inspection. Before your next PPE purchase, ensure your supplier meets these critical requirements for USP800 Standard compliance.
Pre-Purchase Verification Checklist
Use this checklist when evaluating PPE suppliers for Hazardous Drugs Handling compliance
Does the report show both 'Breakthrough Time' AND 'Lag Time'?
Medtecs discloses BOTH metrics for full transparency on permeation curves. Many competitors only report Breakthrough Time, hiding the early detection phase. Carmustine breakthrough must be > 30 min.
Is the gown material low-linting for ISO 7 classified rooms?
Low-linting cleanroom supplies protect HEPA filter integrity in C-SEC environments. Request particle shedding test data.
Are Yellow bins (trace) and Black bins (bulk) correctly specified for disposal of worn PPE?
Ensure compliance with Daniels' Sharpsmart protocols and EPA hazardous waste requirements for Disposing of HD.
Does the supplier have multi-regional production capacity?
Diversified manufacturing reduces supply disruption risk for critical lab supplies. Medtecs: 3 countries (Philippines, Cambodia, China).
Are chemotherapy gloves tested for compatibility with lab equipment cleaning agents?
Verify ASTM D6978 certification and disinfectants compatibility for double-gloving protocols per safety precautions.
Can the supplier provide CoC, test reports, and audit documentation?
Essential for JCI Audit, State Board of Pharmacy inspections, and OSHA compliance. Request QR-linked lot traceability.
Resilient Supply Chain: 3-Country Redundancy
Production redundant across 3 countries to immunize your hospital against geopolitical disruptions and ensure uninterrupted supply of lab supplies and cleanroom supplies:
Philippines
Primary Manufacturing
Cambodia
Glove Production (RMKH)
China
Backup Capacity
Understanding Test Data: Lag Time vs. Breakthrough Time
Lag Time (Detection Time)
The time from initial chemical contact until the first detectable permeation occurs. Medtecs reports this for full transparency.
Breakthrough Time
The time until permeation rate reaches 0.01 µg/cm²/min (ASTM D6978 threshold). This is the critical safety metric.
Medtecs reports BOTH metrics — providing a transparent view of the material’s true barrier performance curve.
USP <800> Implementation Guide
Complete guide for Hazardous Drugs Handling compliance
ASTM Test Report Summary
19-drug permeation test results with Lag Time & Breakthrough data
Donning/Doffing Procedure Poster
Visual guide for Safe-Handling Adherence training
Audit Checklist Template
Ready-to-use checklist for JCI and OSHA inspections
Includes CoC templates, ASTM test reports, compliance documentation, and audit checklists
Regulatory & Professional References
Designed in alignment with Oncology Nursing Society (ONS) guidelines for PPE usage during administration and disposal, and Expert Panel Discussion recommendations.
Main Oncology Solution:
Related USP 800 PPE Resources:
I. Compliance & Strategic Facility ManagementThe Definitive Guide to USP 800 PPE Compliance for Oncology Facilities
Essential Steps for USP 800 PPE Procurement
Mitigating Liability with USP 800 PPE Compliance
Essential Steps for Future-Proofing Your Oncology Facility with PPE Standards
The Business Case for Safety: Justifying Investment in USP 800 PPE
Global Perspectives on USP 800 PPE in Oncology
Steps to Implement USP 800 Guidelines for Oncology Pharmacists
How to Effectively Implement USP 800 PPE Standards in Oncology Nursing
Advanced Protection: Choosing Chemotherapy Gowns and Gloves under USP 800
The Science of Safety: Choosing USP 800 PPE Fabrics
Mastering USP 800 Guidelines for Safe Drug Handling
Optimizing Pharmacy Compounding Environments with USP 800 PPE
Master USP 800 PPE Strategies for Safe Spill Control
Mastering Environmental Safety with USP 800 Decontamination
Mastering Safe Disposal of Hazardous Waste in Healthcare
How to Ensure Effective Respiratory Protection Under USP 800 Standards
Integrating PPE and Engineering Controls for USP 800 Compliance
USP <800> Compliant Hand Protection Solutions
Chemotherapy-tested gloves are the most frequently contacted PPE in hazardous drug handling. USP <800> mandates Double Gloving for all compounding and administration tasks. Medtecs N1000 Nitrile Series provides ASTM D6978 tested protection as the foundation of your hand protection protocol.
⚠️ Procurement Warning: Not All “Exam Gloves” Are Equal
Many facilities mistakenly use standard ASTM D6319 exam gloves for chemotherapy handling. This is non-compliant with USP <800>. Only gloves tested to ASTM D6978(chemotherapy-specific permeation standard at 35°C body temperature) are acceptable for antineoplastic drugs.
Medtecs N1000 Series Specifications
Technical Specifications

Finger-Textured Design
Why N1000? Standard gloves fail as skin heats up. Medtecs N1000 series is tested at 35°C (Body Temperature Simulation) rather than standard room temp (23°C), ensuring the ASTM D6978 chemical barrier holds firm during real-world oncology operations.
Precision grip on glass vials and syringes without compromising the chemical barrier. Critical for Containment Aseptic Compounding Isolators where tactile feedback is essential.
Micro-Textured
Fingertip Pattern
4-5 mil
Optimal Thickness
ASTM D6978 Breakthrough Times
Tested at 35°C (body temperature simulation)
| Drug | Breakthrough | Status |
|---|---|---|
| Carmustine Highest permeation challenge | > 30 min | Pass |
| Thiotepa High lipophilicity | > 30 min | Pass |
| Cyclophosphamide Common alkylating agent | > 240 min | Pass |
| Cisplatin Nephrotoxic platinum compound | > 240 min | Pass |
| Doxorubicin Anthracycline cardiotoxin | > 240 min | Pass |
| Paclitaxel Taxane microtubule inhibitor | > 240 min | Pass |
| 5-Fluorouracil Antimetabolite | > 240 min | Pass |
| Methotrexate Folate antagonist | > 240 min | Pass |
| Etoposide Topoisomerase inhibitor | > 240 min | Pass |
Note: Carmustine and Thiotepa are the most challenging due to their small molecular size and high lipophilicity. > 30 min is the minimum ASTM D6978 requirement; Medtecs N1000 exceeds this threshold.
USP <800> Double Gloving Protocol by Scenario
Recommended chemotherapy gloves configuration for healthcare personnel
| Scenario | Inner Glove | Outer Glove | Change Frequency |
|---|---|---|---|
| Sterile Compounding (USP <797>/<800>) | N1000 (Chemotherapy-tested) under gown cuff | ASTM D6978 compliant glove over cuff (sterile-ready options available) | Every 30 min or immediately if compromised |
| Non-Sterile Compounding | N1000 (Size M) under gown cuff | N1000 (Size L) over gown cuff | Every 30 min or between patients |
| Administration (Infusion Nurses) | N1000 tucked under gown | N1000 extending over gown cuff | Between each patient |
| Spill Response (>5ml) | N1000 Nitrile (chemical barrier) | Utility-grade Chemical Resistant Glove | After spill cleanup complete |
Pro Tip: Using a larger size for the outer glove (e.g., inner M + outer L) reduces hand fatigue during extended compounding sessions—a practice endorsed by experienced oncology pharmacists.
Choosing the Right Barrier Protection:
Medtecs Solution Matrix: Tiered Protection for Every Role
Pharmacy Directors need a tiered approach. From pharmacy techs compounding daily to spill response teams handling waste containment systems—choose the right protection level based on drug categories and workflow requirements.

Tier 1
Compounding
Tier 2
Surgery/HIPEC
Tier 3
Spill/Waste
IL-4036YKTP (Yellow)
Oncology Pharmacists, Pharmacy Techs
Specifications
Engineered PPSB+PE Laminate: Moisture-Vapor Breathable Technology (MVTR) optimizes thermal comfort while maintaining full barrier protection against oral chemo and routine admixtures.
Use Case
Daily Admixture in C-SCA, Handling Oral Chemotherapy, Virtual Hybrid IV Certification.
Key Features
Permeation Test Results*
🧤 The Perfect Pair: Gown + N1000 Gloves
Under-and-Over Technique: Larger outer size reduces hand fatigue
IL-4063WKTP (White)
Surgeons, OR Nurses
Specifications
63gsm Heavy Duty, AAMI Level 4, ASTM F1671 Viral Barrier.
Use Case
HIPEC Surgery, Trauma with Chemo Patients, High-fluid procedures.
Key Features
Permeation Test Results*
🧤 The Perfect Pair: Gown + N1000 Gloves
Under-and-Over Technique: Maintains sterile field + chemo protection
IL-3063WEHTP (Coverall)
EVS Staff, Spill Response Teams
Specifications
Type 5-B/6-B, Hooded, Taped Seams, Full body coverage. Compatible with Daniels’ Sharpsmart protocols.
Use Case
Cytotoxic Drug Spills (>5ml), Waste Disposal, Disposing of HD. 100% Polyolefin material ensures Eco-Friendly Incineration (No toxic fumes).
Key Features
Permeation Test Results*
🧤 The Perfect Pair: Gown + N1000 Gloves
Under-and-Over Technique: Outer protects against sharps (broken glass)
Mandatory Add-ons (USP <800>)
Product Specification Comparison Table
| Specification | IL-4036YKTP | IL-4063WKTP | IL-3063WEHTP |
|---|---|---|---|
| Product Type | Isolation Gown | Isolation Gown | Coverall |
| Weight (gsm) | 36 | 63 | 63 |
| Material | PPSB+PE | PPSB+PE | SMS+PE |
| AAMI Level | Level 3 | Level 4 | N/A |
| Permeation Resistance (19 drugs)* | >480 min | >480 min | >480 min |
| ASTM F1671 (Viral) | — | ✓ Pass | ✓ Pass |
| Type 5-B/6-B | — | — | ✓ Yes |
| Heat-Sealed Seams | ✓ Yes | ✓ Yes | ✓ Yes |
| Primary Use Case | Compounding, Administration | HIPEC, Surgery, Trauma | Spill Control, Waste Disposal |
Medtecs Group — Global Custom Isolation Gown Manufacturing & Factory OEM/ODM Services
-
BY Protection Level (AAMI) Isolation Gowns:
AAMI Level 1 Isolation Gowns,
AAMI Level 2 Isolation Gowns,
AAMI Level 3 Isolation Gowns,
AAMI Level 4 Isolation Gowns,
Yellow AAMI Level 4 Isolation Gowns
-
BY Color Isolation Gowns:
Blue Isolation Gowns,
White Isolation Gowns,
Yellow Isolation Gowns
-
BY Sterility Isolation Gowns:
Non-Sterile Isolation Gowns
-
BY Feature Isolation Gowns:
Isolation Gowns with Knit Cuffs,
Tie Neck Isolation Gowns,
Isolation Gowns with Thumb Loops
-
BY Compliance Standard Isolation Gowns:
FDA 510(k) Cleared Isolation Gowns,
ASTM F1670 Compliant Isolation Gowns (Synthetic Blood),
ASTM F1671 Compliant Isolation Gowns (Viral Penetration)
-
BY Fabric Weight (GSM) Isolation Gowns:
25 gsm Isolation Gowns,
36 gsm Isolation Gowns,
63 gsm Isolation Gowns
-
BY Back Style Isolation Gowns:
Open Back Isolation Gowns,
Over-the-Head Design Isolation Gowns
-
BY Material Isolation Gowns:
SMS Isolation Gowns,
PE Coated Isolation Gowns,
Microporous Laminate Isolation Gowns
-
BY Usability Isolation Gowns:
Disposable Isolation Gowns
-
BY Seam Style Isolation Gowns:
Isolation Gowns with Taped Seams
-
BY Application / Use Case Isolation Gowns:
Isolation Gowns for ICU(Intensive Care Unit),
Isolation Gowns for ER (Emergency Room) & Trauma,
Isolation Gowns for Decontamination,
Isolation Gowns for Basic Patient Care & Visitor Use,
Isolation Gowns for Long-Term Care Facilities
-
BY Solution :
ER & Trauma Center PPE: Impervious Gowns for High-Risk Surgery
(Trauma Surgeons,
Triage Nurses,
ICU Staff,
Procurement
),USP Oncology PPE: Gowns & Gloves >480 Min Carmustine Protection
(Pharmacy,
Nursing,
EVS,
Procurement
),Hospital CSSD Decontamination Biosafety Compendium
(Technicians,
Compliance Officers,
OHS Personnel,
Procurement
)



